When Asi Naim, a severely autistic Israeli boy, started smacking his head against the wall and hurting himself in other ways, his parents tried every kind of psychiatric drug to calm him. Nothing worked.
“He was so totally out of it,” said his mother, Ricky Naim Blumenfeld. “It was scary.”

Asi Naim, left, and family
Photographer: Asher Blumenfeld/RAPhotography24
Four years later, Asi loves music, being at parties, going to the movies and traveling abroad.
The same medication has helped many of the 60 autistic children enrolled in neuro-pediatrian Adi Aran’s program. Aran is now in the middle of a second, controlled study with 100 children. The end goal: approval by the U.S. Federal Drug Administration as an experimental treatment.
The agency hasn’t yet approved a botanically derived medical cannabis product. But classifying the formulation as an FDA-recognized drug would mean it’s no longer covered by the federal ban on U.S. imports of marijuana, a key step in solidifying Israel’s reputation as a global center for medical weed research, development and exports. Belgium, Netherlands, Romania, Portugal, Czech Republic, Germany and Switzerland are major potential markets for medical marijuana, says Ameri Research Inc., a U.S. market-research and consulting company.
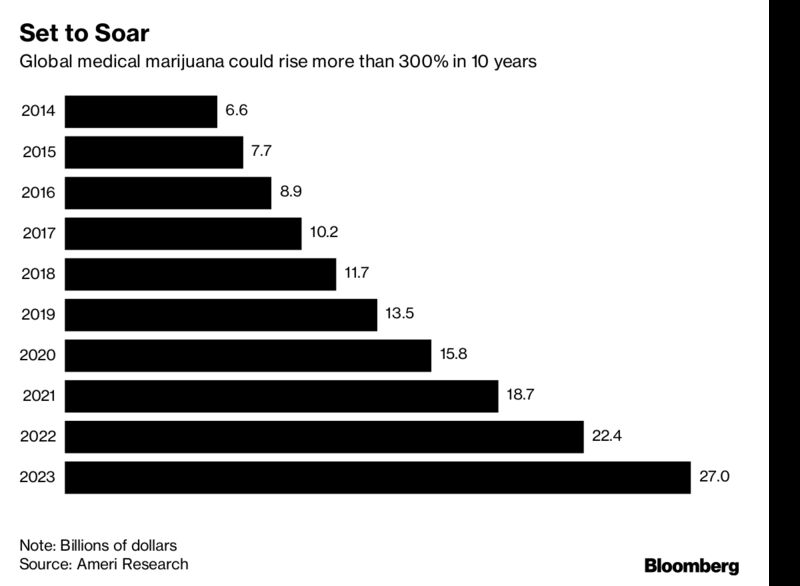
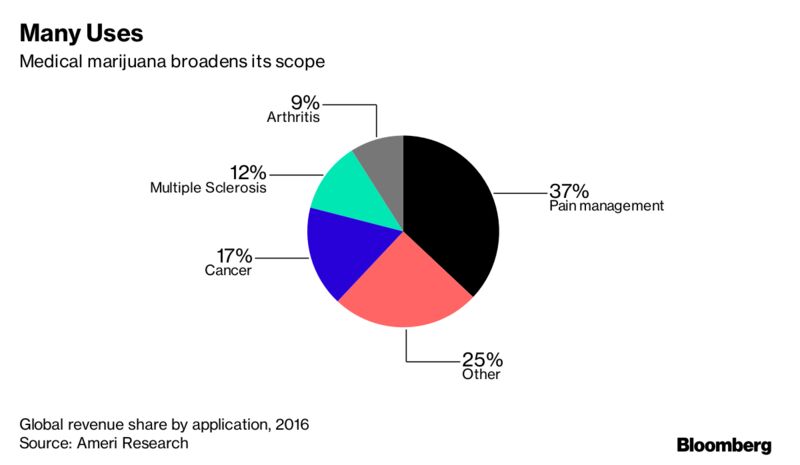
The market for medical weed will reach $33 billion in 2024, Ameri said in an April report. That’s a threefold increase from projected sales of $10 billion this year amid growing acceptance of marijuana for pain management, multiple sclerosis, cancer, arthritis and other chronic conditions.
Israeli grower and medicinal formula developer Breath of Life Pharma, which supported the autism research, plans to apply for “investigative new drug” status from the FDA next year if the results from Aran’s trials are conclusive enough. FDA recognition would boost a company that plans to open hubs for production, cultivation and clinical trials in several countries outside the U.S., said Chief Executive Officer Tamir Gedo.
Israel won’t be the only country selling its cannabis on the global market. Canada and the Netherlands already do so. Uruguay, which allows government-authorized medical cannabis exports, and Colombia, which in 2015 established a framework for cultivation, processing, research and development and export of medical cannabis, are expected to start sales abroad in the near future.
Israeli Brand
The Israeli government is counting on its products standing out, distinguished by agricultural technology created to make the desert bloom, such as Netafim Ltd.’s drip-irrigation system and seed company Hazera Ltd.’s specially bred crops that include tomatoes, radishes and melons.“When Israel handed out original licenses for growing cannabis, they started with an advanced agricultural infrastructure first used to grow other leafy green vegetables and fruit,” said Scott Greiper, founding partner of Viridian Capital Advisors, a New York-based financial advisory firm dedicated to the cannabis industry.
The government team recommending export said Israeli companies must maintain high, medical-grade standards and be subject to strict supervision, with sales allowed only to countries that specifically approved Israel’s merchandise.
Still, there’s a limited window of opportunity to Israel’s advantage in the market. As other countries begin producing for their own markets, the need for imports could fall, said Saul Kaye, founder of iCAN, a company that invests in the local cannabis market.
The Health Ministry plans to increase licenses for cannabis growers from eight to nearly 50 and has invested 100 million shekels in research and development. The Ministry of Health has approved 150 research proposals, 35 of them clinical trials. More than 50 U.S. companies are doing medical marijuana research in Israel and in 2016 invested more than $125 million in Israeli cannabis operations, according to iCAN.
First and second-time civilian offenders caught with cannabis for recreational use are fined and won’t necessarily have criminal records, according to a March cabinet decision.
With the export law expected to pass early in 2018, Breath of Life is preparing for mass production.
This year it bought Syngenta AG’s former factory in Gedera, south of Tel Aviv, to boost its harvest of plants and medicines. As part of his bid to insure pharma-standard products, CEO Gedo has hired former researchers from the generic medicine maker Teva Pharmaceutical Industries Ltd.; they now make up about 25 percent of his workforce.
Today Asi’s little brother Inbar, who is 15 and also on the autism spectrum, takes medical cannabis to relieve anxiety. He now is able invite friends over and sometimes even hug them.
The life of the entire family has changed.
“We go to plays, to the library, the cafe at the beach,” said Naim Blumenfeld, their mother. “We do normal things.”

No comments:
Post a Comment